In school, we were taught about acids and bases and their different characteristics. However, if you have been out of school for a bit and are not working in the field of science, it can be difficult to tell what exactly differentiates the two. In this article, we will explore the difference between an acid and a base.
Summary Table
| Acid | Base |
| Releases hydrogen ions and donates protons when combined with water | Absorbs hydrogen ions and accepts protons when combined with water |
| Sour taste | Soapy taste |
| pH level is below 7 | pH level is above 7 |
| Can turn litmus paper to red | Can turn litmus paper to blue |
| Corrosive to metals | Can denature protein |
Descriptions
An acid is a chemical compound that releases hydrogen ions and donates protons when dissolved in water. It has a sour taste (although it is important to note that not all types of acid can be tasted) and is innately corrosive to metals, even gold.
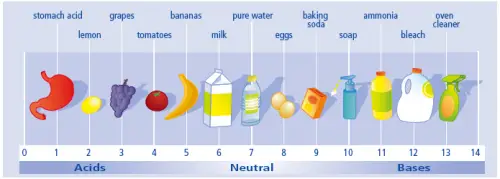
Acid causes litmus paper to turn red. Its pH level is below 7. The lower the pH value, the stronger the acid. When combined in equal amounts with a base, acid loses its acidic characteristic, a process called neutralization.
Although acid is dangerous to the human body in extensive amounts, some types of acid are safe to use and can be found in common items you see every day. Common examples of acids are:
- Acetic acid – the type of acid found in vinegar
- Lactic acid – found in sour cream, milk, and cheese
- Citric acid – the acid naturally found in citrus fruits such as oranges, lemon, and lime
- Sulphuric acid – commonly used in fertilizers
- Salicylic acid – used in topical treatments for various skin problems
- Hydrochloric acid – the acid found in our stomachs that breaks down food
- Muriatic acid – used to clean tough stains
On the contrary, a base is a chemical compound that absorbs hydrogen ions. When mixed with water, it releases hydroxide ions and accepts protons. It has a soapy taste (note that it is not safe for humans to taste or touch some types of bases) and can be slippery to the touch.
A base can turn litmus paper to blue. Its pH level is higher than 7. The higher the pH level the stronger the base.
Bases have the capability to denature protein, which is why they are dangerous to humans when used extensively. However, some types are commonly used in the manufacturing industry, in the kitchen or at home as cleaning products. Examples include:
- Ammonia – commonly used as a household cleaner
- Baking soda – also called sodium bicarbonate; used in the kitchen and as a homemade cleaning agent
- Sodium hydroxide – also called caustic soda; is used in making paper, soap, and petroleum products
- Lithium hydroxide – used in manufacturing batteries
- Calcium hydroxide – also called slaked lime; used in food preparation
Acid vs Base
What, then, is the difference between an acid and a base?
In terms of chemical reactions, acids release hydrogen ions and donate protons when combined with water. Bases, on the other hand, absorb hydrogen ions and accept protons.
Acids have a sour taste and a pH level of less than 7. Acid can turn litmus paper to red. When combined with a base, its acidity is lost. It is highly corrosive, even to gold.
On the contrary, bases have a soapy taste and a pH level of above 7. A base can turn litmus paper to blue. It has the capability to denature protein.



