A cation and an anion are ions, which are electrically-charged groups of atoms that carry either a positive or a negative charge. Despite their strong association, they are far from being interchangeable. This article draws the line between the two.
Summary Table
| Cation | Anion |
| Originated from the Greek word κάτω (káto), meaning “down” | Originated from the Greek word ἄνω (ánō), which means “up” |
| A positively-charged ion | A negatively-charged ion |
| Made up of more protons than electrons | Made up of more electrons than protons |
| Smaller | Larger |
|
Ionic radius is lower than 0.8 × 10−10 m (0.8 Å) |
Ionic radius is over 1.3 × 10−10 m (1.3 Å) |
|
Attracted to cathodes during electrolysis |
Attracted to anodes during electrolysis |
|
In writing a chemical compound, a cation is always written before an anion |
In writing a chemical compound, an anion is always written after a cation |
|
Symbol: “+” |
Symbol: “–” |
Definitions
A cation is a positively-charged ion. A particle that can be present in metallic and non-metallic elements, a cation pertains to a group of compounds formed when an atom loses one or more negatively-charged subatomic particles, which are known as electrons.
Meanwhile, an anion is a negatively-charged ion. It is formed when atoms or molecules contain more electrons than protons, which are positively-charged subatomic particles.
Cation vs Anion
Even though both are ionic particles, there is a huge difference between a cation and an anion.
Etymology
First proposed by William Whewell in the year 1834, the term “anion” originated from the Greek word ἄνω (ánō), which means “up.” Cation, on the other hand, was coined from the Greek word κάτω (káto), meaning “down.” Both terms pertain to the movement of the ions during electrolysis.
Charge
A cation is a positively-charged ion, while an anion is negatively-charged.
Composition
A cation is formed when an atom loses one or more negatively-charged subatomic particles, which are known as electrons. Unlike an anion, it is made up of more protons than electrons. An anion, by contrast, carries extra electrons.
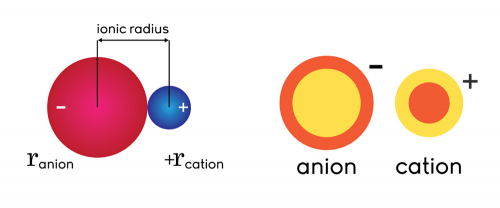
Size
The size of a cation and an anion is defined by their ionic radius. Since anions carry extra electrons, they are typically larger than cations. The space within a crystal, for instance, is mostly occupied by anions, while cations fill in the gaps between these spaces.
To compare, the ionic radius of a cation is usually lower than 0.8 × 10−10 m (0.8 Å), while an anion’s radius is over 1.3 × 10−10 m (1.3 Å).
Electrolysis
Electrolysis is a process where ionic particles are broken down by the use of electricity. During electrolysis, anodes, which are positively-charged electrodes, attract anions. Cathodes, on the other hand, are negatively-charged electrodes that attract cations.
Chemical Formula
When writing a chemical compound, the element symbol for the cation is always written before the anion. For instance, sodium chloride contains NA+ (a cation) and CL- (an anion), and is thereby written as NaCl.
Another notable difference is the symbols used for each ionic particle. While element symbols for cations are usually followed by a “+”, anions are represented by an element symbol and a “–” sign.
These symbols also describe the number of electrons and protons in a compound. If, for example, the symbol is accompanied by “-2”, it means that the chemical compound has 2 fewer protons than electrons. If, by contrast, the symbol is followed by “+2”, it directly implies that the the compound has 2 fewer electrons than protons.



