Voltaic or galvanic cells and electrolytic cells are electrochemical cells. Electrochemical cells are devices that convert between chemical and electrical energy. There are a variety of applications associated with electrochemical cells. Voltaic or galvanic cells and electrolytic cells are words we don’t hear every day but they are something that we constantly use. In this article, we will define them and describe how they differ.
Summary Table
| Voltaic or Galvanic Cells | Electrolytic Cells |
| Converts chemical energy to electrical energy | Converts electrical energy into chemical energy |
| Produces electricity from a chemical reaction | Induces chemical reaction from electrical energy |
| Redox reaction is spontaneous | Redox reaction is induced from an external electrical energy source |
| Anode is the negative electrode | Anode is the positive electrode |
| Cathode is the positive electrode | Cathode is the negative electrode |
Descriptions
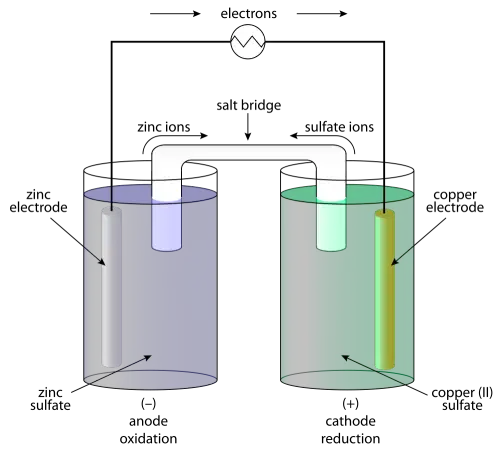
A voltaic cell is an electrochemical device that produces electrical energy from a spontaneous reduction-oxidation reaction, also called a redox reaction. A redox reaction is a chemical reaction wherein one reactant loses electrons (oxidation reaction) and the other reactant gains electrons (reduction reaction).
The voltaic cell is usually made of two different metals; each submerged separately in ionic solutions of the respective metal and connected together by a salt bridge. The metals are called electrodes. One electrode is called the anode and is the site where the oxidation process occurs. Since the reactant on this electrode release electrons, there will be a buildup of negative charge during the reaction.
On the voltaic cell, the anode is also called the negative electrode. The other electrode is called the cathode and it is where the reduction reaction occurs. On this electrode, the reactant consumes electrons, leaving a net positive charge. The cathode then is called the positive electrode for voltaic cells. Because of this buildup of charges on each electrode, connecting the electrodes with a conductor completes the circuit and initiates current flow across the circuit.
The voltaic cell derives its name from the Italian scientist Alessandro Volta. Volta developed the first battery, which consisted of a stack of copper and zinc discs separated by cardboard soaked in salt water. Strictly speaking, a battery is defined as a group of more than one individual voltaic cell, electrically connected in series.
Each unit of copper-cardboard-zinc is a single cell, and the voltaic stack is the battery.
Applications include alkaline dry cell batteries, which use Manganese Dioxide (MnO2) as the cathode and Zinc (Zn) as the anode. Mobile phones typically use lithium ion batteries, which use Lithium as the anode.
Another example of a voltaic cell is the lead acid battery, most commonly used as car batteries. These use lead (Pb) as the anode and lead dioxide (PbO2) as the cathode, with sulfuric acid (H2SO4) as the electrolytic solution.
A galvanic cell is just a different name for voltaic cell and can be used interchangeably. It derives its name from another Italian scientist, Luigi Galvani. He was the first to discover that when two different metals connected to each other touch different points on a frog’s leg nerves at the same time, it can make the leg muscles contract. This led the way for Volta to investigate and develop the voltaic pile mentioned earlier.
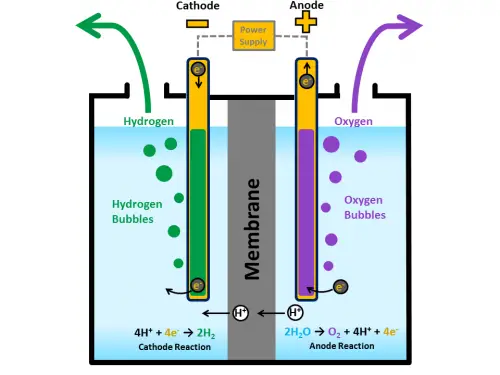
An electrolytic cell is a device that induces redox reactions by applying electrical energy. An electrolytic cell is typically composed of two electrodes and an aqueous solution of dissolved ions, also called the electrolyte. In an electrolytic cell, the voltage applied across the electrodes forces the redox reaction when ions in the electrolyte are attracted to the electrode of opposite charge. In electrolytic cells, the cathode carries the negative charge, while the anode is the positive charge. This is because the reduction reaction occurs when electrons are forced into the system by an external electrical source, making the ions gain electrons. By definition, the cathode is the electrode where the reduction process happens. The opposite is true for the other electrode, the anode, where the oxidation reaction happens. An external voltage source is always required to initiate and sustain the redox reaction in an electrolytic cell.
The typical use for electrolytic cells is electrolysis, an example of which is the separation of water into oxygen and hydrogen. During the electrolysis process, oxygen bubbles form at the anode, while hydrogen forms at the cathode. Another practical use is for electroplating. The metal that is being plated is placed at the cathode and the metal used for plating is placed at the anode. The dissolved ions of the plating metal are deposited on the target metal at the cathode. Rechargeable batteries act as voltaic cells when discharging, but when recharging, they act as electrolytic cells.
Voltaic or Galvanic Cells vs Electrolytic Cells
What is the difference between voltaic or galvanic cells and electrolytic cells? Voltaic or galvanic cells convert chemical energy to electrical energy and produces electricity from a chemical reaction. Electrolytic cells, on the other hand, convert electrical energy into chemical energy thereby inducing a chemical reaction from electrical energy. The redox reaction in voltaic or galvanic cells is spontaneous while the redox reaction in electrolytic cells is induced from an external electrical energy source. In voltaic or galvanic cells, the anode is the negative electrode and the cathode is the positive electrode. Conversely, in electrolytic cells, the anode is the positive electrode and the cathode is the negative electrode.